| Source: | Rabbit | Gene Id: | 55662 |
| Isotype: | IgG | Swiss Prot: | Q9NWT6 |
| purity: | Affinity purification |
| Background: |
| FIH (Factor inhibiting HIF-1, HIF asparagine hydroxylase) is a dioxygen-dependent asparaginyl hydroxylase that modifies target protein function by hydroxylating target protein asparagine residues. Hypoxia-inducible factor (HIF), a transcriptional activator involved in control of cell cycle in response to hypoxic conditions, is an important target for FIH regulation. FIH functions as an oxygen sensor that regulates HIF function by hydroxylating at Asn803 in the carboxy-terminal transactivation domain (CAD) of HIF. During normoxia, FIH uses cellular oxygen to hydroxylate HIF-1 and prevent interaction of HIF-1 with transcriptional coactivators, including the CBP/p300-interacting transactivator. Under hypoxic conditions, FIH remains inactive and does not inhibit HIF, allowing the activator to regulate transcription of genes in response to low oxygen conditions. FIH activity is regulated in through interaction with proteins, including Siah-1, which targets FIH for proteasomal degradation. The Cut-like homeodomain protein CDP can bind the FIH promoter region to regulate FIH expression at the transcriptional level. Phosphorylation of HIF at Thr796 also can prevent FIH hydroxylation on Asn803. Potential FIH substrates also include proteins with ankyrin repeat domains, such as Iκ-B, Notch, and ASB4. |
| Reactivity | Human, Mouse, Rat |
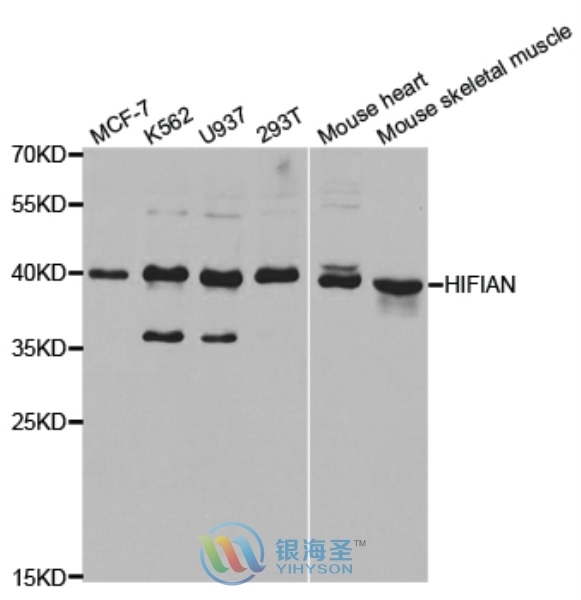
| Tested applications | WB IHC IP IF ChIP |
| Clonality | Polyclonal Antibody |
| Calculated MW | 40 kDa |
| Recommended Dilutions |
WB 1:500-1:2000
IHC 1:50-1:200
IF 1:50-1:200
ChIP 1:20-1:100
Dot 1:20-1:100
|
| Immunogen | A recombinant protein of human HIF1AN |
| Storage | Store at -20°C or -80°C in PBS with 0.02% sodium azide and 50% glycerol. Avoid freeze/thaw cycles. |
| Synonym | FIH1 |
沪ICP备15003525号-1 Copyright ©, 2013-2022, Yihyson All Rights Reserved.