| Source: | Rabbit | Gene Id: | 64135 |
| Isotype: | IgG | Swiss Prot: | Q9BYX4 |
| purity: | Affinity purification |
| Background: |
| Antiviral innate immunity depends on the combination of parallel pathways triggered by virus detecting proteins in the Toll-like receptor (TLR) family and RNA helicases, such as Rig-I (retinoic acid-inducible gene I) and MDA-5 (melanoma differentiation-associated antigen 5), which promote the transcription of type I interferons (IFN) and antiviral enzymes (1-3). TLRs and helicase proteins contain sites that recognize the molecular patterns of different virus types, including DNA, single-stranded RNA (ssRNA), double-stranded RNA (dsRNA), and glycoproteins. These antiviral proteins are found in different cell compartments; TLRs (i.e. TLR3, TLR7, TLR8, and TLR9) are expressed on endosomal membranes and helicases are localized to the cytoplasm. Rig-I expression is induced by retinoic acid, LPS, IFN, and viral infection (4,5). Both Rig-I and MDA-5 share a DExD/H-box helicase domain that detects viral dsRNA and two amino-terminal caspase recruitment domains (CARD) that are required for triggering downstream signaling (4-7). Rig-I binds both dsRNA and viral ssRNA that contains a 5'-triphosphate end not seen in host RNA (8,9). Though structurally related, Rig-I and MDA-5 detect a distinct set of viruses (10,11). The CARD domain of the helicases, which is sufficient to generate signaling and IFN production, is recruited to the CARD domain of the MAVS/VISA/Cardif/IPS-1 mitochondrial protein, which triggers activation of NF-κB, TBK1/IKKε, and IRF-3/IRF-7 (12-15). |
| Reactivity | Human |
| Tested applications | WB IHC IF |
| Clonality | Polyclonal Antibody |
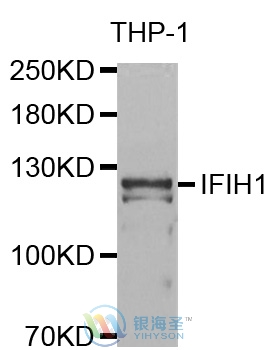
| Calculated MW | 117 kDa |
| Recommended Dilutions |
WB 1:500-1:2000
IHC 1:50-1:200
IF 1:50-1:200
|
| Immunogen | A recombinant protein of human IFIH1 |
| Storage | Store at -20°C or -80°C in PBS with 0.02% sodium azide and 50% glycerol. Avoid freeze/thaw cycles. |
| Synonym | Hlcd, IDDM19, MDA-5, MDA5, MGC133047 |
沪ICP备15003525号-1 Copyright ©, 2013-2022, Yihyson All Rights Reserved.