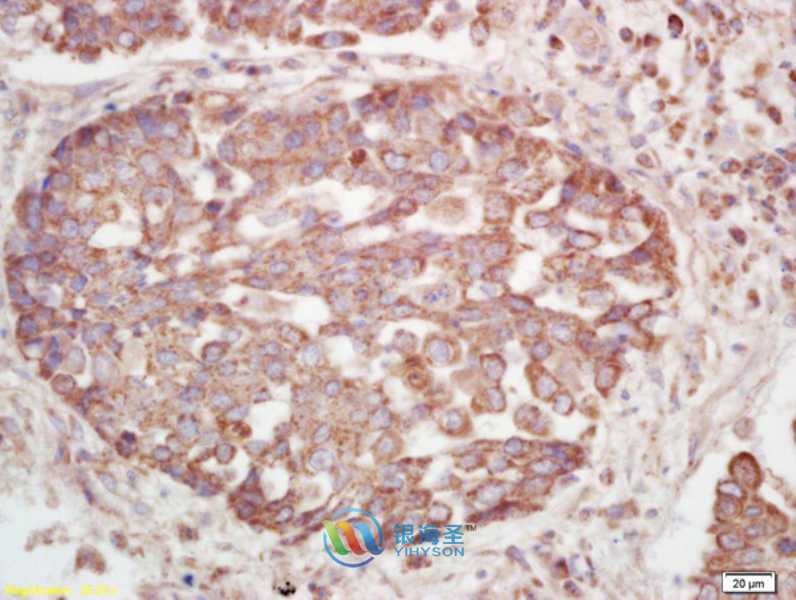
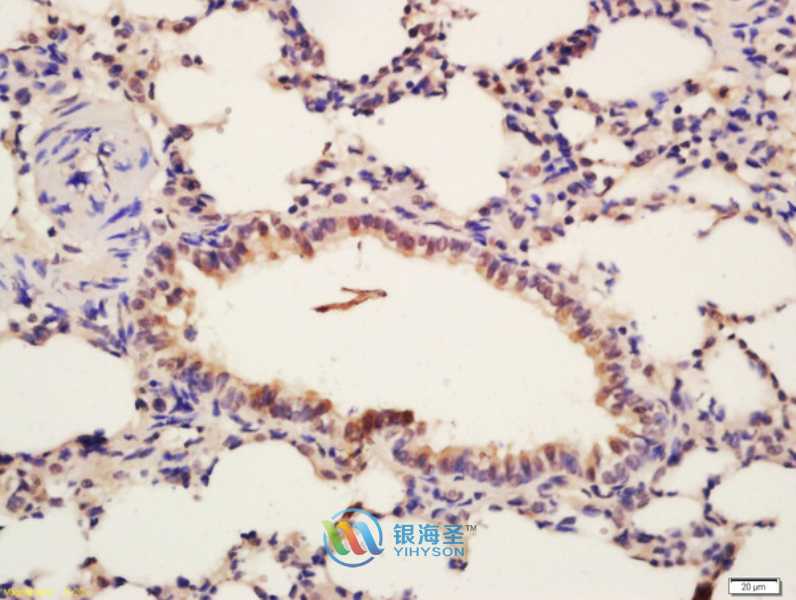
YRP05458P-01
[Polyclonal Antibody]
Phospho-Insulin Receptor Beta (Tyr1185) Rabbit Polyclonal Antibody

www.yhsbio.com
market@yhsbio.com
support@yhsbio.com
+86-21-54651191
Room 703,Building 6,333# Guiping
Rd.,Xuhui District,Shanghai,China
market@yhsbio.com
support@yhsbio.com
+86-21-54651191
Room 703,Building 6,333# Guiping
Rd.,Xuhui District,Shanghai,China
DATASHEET
| Species: | Rabbit |
| Applications: | WB IHC IF |
| Immunogen Range: | KLH conjugated synthetic phosphopeptide derived from human INSR around the phosphorylation site of Tyr1185 [DI(p-Y)ET] |
| Clonality: | Polyclonal Antibody |
| Isotype: | IgG |
| GENE ID: | 3643 |
| Swiss Prot: | P06213 |
| Synonyms: | HHF5, CD22, Insulin receptor, IR, INSR |
| Purification: | Purified by Protein A. |
| Storage: | Aqueous buffered solution containing 100ug/ml BSA, 50% glycerol and 0.09% sodium azide. Store at -20℃ for 12 months |
| Background: | The human insulin receptor is a heterotetrameric membrane glycoprotein consisting of disulfide linked subunits in a beta-alpha-alpha-beta configuration. The beta subunit (95 kDa) possesses a single transmembrane domain, whereas the alpha subunit (135 kDa) is completely extracellular. The insulin receptor exhibits receptor tyrosine kinase (RTK) activity. RTKs are single pass transmembrane receptors that possess intrinsic cytoplasmic enzymatic activity, catalyzing the transfer of the gamma phosphate of ATP to tyrosine residues in protein substrates. RTKs are essential components of signal transduction pathways that affect cell proliferation, differentiation, migration and metabolism.Included in this large protein family are the insulin receptor and the receptors for growth factors such as epidermal growth factor, fibroblast growth factor and vascular endothelial growth factor. Receptor activation occurs through ligand binding, which facilitates receptor dimerization and autophosphorylation of specific tyrosine residues in the cytoplasmic portion. The interaction of insulin with the alpha subunit of the insulin receptor activates the protein tyrosine kinase of the beta subunit, which then undergoes an autophosphorylation that increases its tyrosine kinase activity. Three adapter proteins, IRS1, IRS2 and Shc, become phosphorylated on tyrosine residues following insulin receptor activation. These three phosphorylated proteins then interact with SH2 domain containing signaling proteins. |
| Caculated MW: | / |
| Observed MW: | Refer to Figures |
| Applications: |
WB 1:100-1:1000 IHC 1:100-1:500 IF 1:50-1:200 |
| Reacitivity: | Human, Mouse, Rat |
For research use only. Not intended for diagnostic or therapeutic use!
Additional information