ARP3055-01
[Polyclonal Antibody]
HCK Rabbit Polyclonal Antibody

www.yhsbio.com
market@yhsbio.com
support@yhsbio.com
+86-21-54651191
Room 703,Building 6,333# Guiping
Rd.,Xuhui District,Shanghai,China
market@yhsbio.com
support@yhsbio.com
+86-21-54651191
Room 703,Building 6,333# Guiping
Rd.,Xuhui District,Shanghai,China
DATASHEET
| Species: | Rabbit |
| Applications: | WB IHC |
| Immunogen Range: | A recombinant protein of human HCK |
| Clonality: | Polyclonal Antibody |
| Isotype: | IgG |
| GENE ID: | 3055 |
| Swiss Prot: | P08631 |
| Synonyms: | JTK9 |
| Purification: | Affinity purification |
| Storage: | Store at -20°C or -80°C in PBS with 0.02% sodium azide and 50% glycerol. Avoid freeze/thaw cycles. |
| Background: | Hck (hemopoietic cell kinase) is a protein tyrosine kinase of the Src family prominently expressed in the lymphoid and myeloid lineages of hemopoiesis (1). It participates in transducing a variety of extracellular signals, which ultimately affect cellular processes including proliferation, differentiation and migration.The well-defined modular structure of Hck comprises a relatively divergent, NH2-terminal "unique" domain, which is subject to post-translational lipid modifications thereby targeting Hck to the plasma membrane. Src homology 3 (SH3) and 2 (SH2) domains, and a tyrosine kinase catalytic domain follow the "unique" domain. The catalytic activity of Hck is regulated, both positively and negatively, by tyrosine phosphorylation of highly conserved tyrosine (Y) residues. Phosphorylation of a single conserved Tyr499 residue in the COOH terminus of Hck by the protein kinase Csk renders Hck inactive as a result of an intramolecular interaction between the phosphorylated tyrosine (pY) residue and its own SH2 domain. Disruption of this interaction, either as a result of dephosphorylation, or substitution of the COOH-terminal regulatory Y residue with phenylalanine (F; e.g., HckY499F), or COOH-terminal truncation mutations as observed in the virally transduced v-Src oncoprotein, results in constitutive activation of Hck. In contrast to phosphorylation of the COOH-terminal regulatory tyrosine residue, autophosphorylation of a tyrosine residue (Tyr388) within the kinase domain of Hck acts to positively regulate its catalytic activity. Thus, activation of Hck requires both disruption of the COOH-terminal regulatory tyrosine-SH2 domain interaction and autophosphorylation of the regulatory tyrosine residue within the kinase domain ( 2, 3). The dysfunction or dysregulation of Hck may contribute to the pathogenesis of some human leukemias (4). |
| Caculated MW: | 57 kDa |
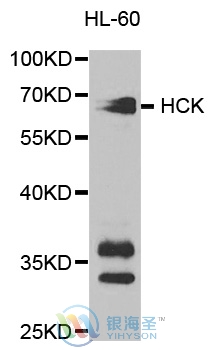
| Observed MW: | Refer to Figures |
| Applications: |
WB 1:500-1:2000 IHC 1:50-1:200 |
| Reacitivity: | Human, Mouse, Rat |
For research use only. Not intended for diagnostic or therapeutic use!
Additional information